Google Scholar and PubMed search results for “”simplified analogue” natural products”, “”simplified analogues” natural products”, “”simplified-analog” natural products”, “”simplified analogs” natural products”, “”function-oriented-synthesis” natural products”, and “pseudo-natural-products”.
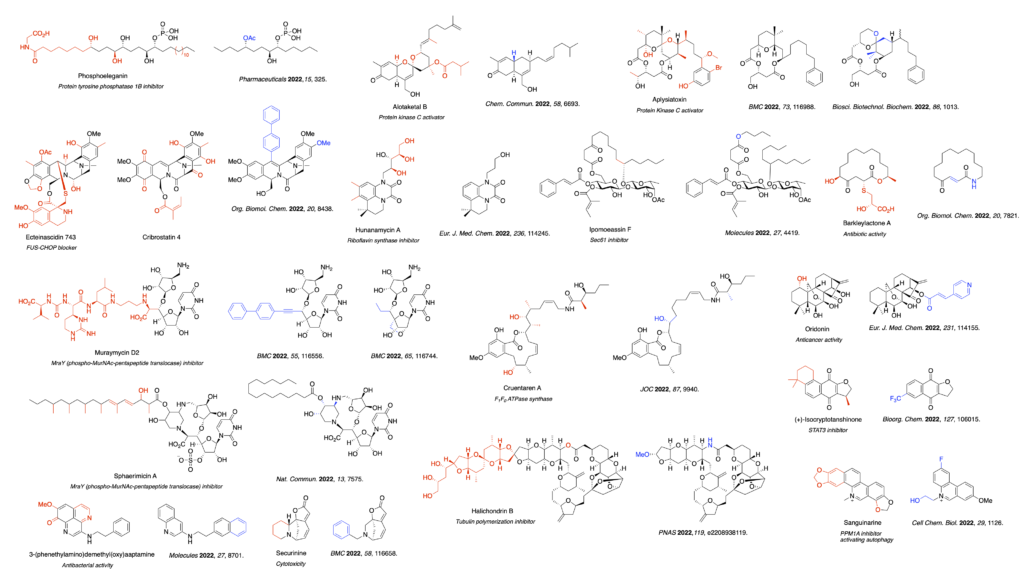
- Casertano M, Genovese M, Piazza L, Balestri F, Del Corso A, Vito A, Paoli P, Santi A, Imperatore C, Menna M. Identifying Human PTP1B Enzyme Inhibitors from Marine Natural Products: Perspectives for Developing of Novel Insulin-Mimetic Drugs. Pharmaceuticals (Basel). 2022 Mar 8;15(3):325. doi: 10.3390/ph15030325. PMID: 35337123; PMCID: PMC8950868.
- Wang M, Yu BB, Yao ZJ. Simplified hybrids of two anticancer bistetrahydroisoquinoline alkaloids ecteinascidin 743 and cribrostatin 4 and inhibitory activity against proliferation of cancer cells. Org Biomol Chem. 2022 Nov 9;20(43):8438-8442. doi: 10.1039/d2ob01707e. PMID: 36254754.
- Okamoto K, Ishikawa A, Okawa R, Yamamoto K, Sato T, Yokota SI, Chiba K, Ichikawa S. Design, synthesis and biological evaluation of simplified analogues of MraY inhibitory natural product with rigid scaffold. Bioorg Med Chem. 2022 Feb 1;55:116556. doi: 10.1016/j.bmc.2021.116556. Erratum in: Bioorg Med Chem. 2022 May 1;61:116689. doi: 10.1016/j.bmc.2022.116689. PMID: 35016115.
- Kusaka S, Yamamoto K, Shinohara M, Minato Y, Ichikawa S. Design, synthesis and conformation-activity relationship analysis of LNA/BNA-type 5′-O-aminoribosyluridine as MraY inhibitors. Bioorg Med Chem. 2022 Jul 1;65:116744. doi: 10.1016/j.bmc.2022.116744. Epub 2022 Apr 20. PMID: 35500521.
- Nakaya T, Yabe M, Mashalidis EH, Sato T, Yamamoto K, Hikiji Y, Katsuyama A, Shinohara M, Minato Y, Takahashi S, Horiuchi M, Yokota SI, Lee SY, Ichikawa S. Synthesis of macrocyclic nucleoside antibacterials and their interactions with MraY. Nat Commun. 2022 Dec 20;13(1):7575. doi: 10.1038/s41467-022-35227-z. PMID: 36539416; PMCID: PMC9768162.
- Maki J, Oshimura A, Tsukano C, Yanagita RC, Saito Y, Sakakibara Y, Irie K. AI and computational chemistry-accelerated development of an alotaketal analogue with conventional PKC selectivity. Chem Commun (Camb). 2022 Jun 9;58(47):6693-6696. doi: 10.1039/d2cc01759h. PMID: 35608215.
- Sekido T, Yamamoto K, Yanagita RC, Kawamani Y, Hanaki Y, Irie K. A simplified analog of debromoaplysiatoxin lacking the B-ring of spiroketal moiety retains protein kinase C-binding and antiproliferative activities. Bioorg Med Chem. 2022 Nov 1;73:116988. doi: 10.1016/j.bmc.2022.116988. Epub 2022 Aug 27. PMID: 36113282.
- Suzuki Y, Moritoki K, Kajiwara M, Yanagita RC, Kawanami Y, Hanaki Y, Irie K. Design, synthesis, and biological activity of a synthetically accessible analog of aplysiatoxin with an (R)-(-)-carvone-based conformation-controlling unit. Biosci Biotechnol Biochem. 2022 Jul 22;86(8):1013-1023. doi: 10.1093/bbb/zbac084. PMID: 35648459.
- Shingare RD, MacMillan JB, Reddy DS. Antibiotic natural product hunanamycin A: Lead identification towards anti-Salmonella agents. Eur J Med Chem. 2022 Jun 5;236:114245. doi: 10.1016/j.ejmech.2022.114245. Epub 2022 Mar 2. PMID: 35421661.
- O’Keefe S, Bhadra P, Duah KB, Zong G, Tenay L, Andrews L, Schneider H, Anderson A, Hu Z, Aljewari HS, Hall BS, Simmonds RE, Helms V, High S, Shi WQ. Synthesis, Biological Evaluation and Docking Studies of Ring-Opened Analogues of Ipomoeassin F. Molecules. 2022 Jul 10;27(14):4419. doi: 10.3390/molecules27144419. PMID: 35889292; PMCID: PMC9320607.
- Dou X, Patel BA, D’Amico T, Subramanian C, Cousineau E, Yi Y, Cohen M, Blagg BSJ. Synthesis and Evaluation of Simplified Cruentaren A Analogues. J Org Chem. 2022 Aug 5;87(15):9940-9956. doi: 10.1021/acs.joc.2c00948. Epub 2022 Jul 27. PMID: 35894845.
- Malatinský T, Valachová D, Pinčeková L, Scherhaufer D, Olejníková P, Májeková M, Vargová J, Gaálová-Radochová B, Bujdáková H, Nováčiková J, Farley AJM, Berkeš D, Jakubec P, Kolarovič A, Caletková O. Synthesis and structure-activity relationship of berkeleylactone A-derived antibiotics. Org Biomol Chem. 2022 Oct 12;20(39):7821-7832. doi: 10.1039/d2ob01452a. PMID: 36169622.
- Shi X, Du TT, Zhang Z, Liu X, Yang Y, Xue N, Jiao X, Chen X, Xie P. (+)-Isocryptotanshinone derivatives and its simplified analogs as STAT3 signaling pathway inhibitors. Bioorg Chem. 2022 Oct;127:106015. doi: 10.1016/j.bioorg.2022.106015. Epub 2022 Jul 10. PMID: 35849894.
- Mukomura J, Nonaka H, Sato H, Kishimoto M, Arai M, Kotoku N. Anti-Mycobacterial N-(2-Arylethyl)quinolin-3-amines Inspired by Marine Sponge-Derived Alkaloid. Molecules. 2022 Dec 8;27(24):8701. doi: 10.3390/molecules27248701. PMID: 36557834; PMCID: PMC9781020.
- Le Roch M, Afonso D, Chirkin E, Guillory X, Porée FH. Structure simplification of the Securinine skeleton reveals the importance of BCD ring system for the cytotoxic activity on HCT116 and HL60 cell lines. Bioorg Med Chem. 2022 Mar 15;58:116658. doi: 10.1016/j.bmc.2022.116658. Epub 2022 Feb 12. PMID: 35183880.
- Berton S, Chen L, Liang YC, Xu Z, Afriyie-Asante A, Rajabalee N, Yang W, Sun J. A selective PPM1A inhibitor activates autophagy to restrict the survival of Mycobacterium tuberculosis. Cell Chem Biol. 2022 Jul 21;29(7):1126-1139.e12. doi: 10.1016/j.chembiol.2022.03.006. Epub 2022 Mar 22. PMID: 35320734.
- Liu J, Xie S, Shao X, Xue S, Du P, Wu H, Xu S, Chen ZS, Yang DH, Xu J, Yao H. Identification of new potent anticancer derivatives through simplifying the core structure and modification on their 14- hydroxyl group from oridonin. Eur J Med Chem. 2022 Mar 5;231:114155. doi: 10.1016/j.ejmech.2022.114155. Epub 2022 Jan 29. PMID: 35121201.
- Nicolaou KC, Pan S, Shelke Y, Rigol S, Bao R, Das D, Ye Q. A unified strategy for the total syntheses of eribulin and a macrolactam analogue of halichondrin B. Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2208938119. doi: 10.1073/pnas.2208938119. Epub 2022 Aug 5. PMID: 35930662; PMCID: PMC9371655.