Since 2010
Peer-Reviewed Papers
*corresponding author
- Six-step synthesis and epithelial–mesenchymal transition-inhibitory activity of a tetralone-based vitetrifolin analog.

Yusuke Hananki*, Yasunori Sugiyama, Ryo C. Yanagita.
Biosci. Biotechnol. Biochem. 2025, 89, 805–810. [DOI: 10.1093/bbb/zbaf030] [Pubmed: 40058878] - Validation of Machine Learning-assisted Screening of PKC Ligands: PKC Binding Affinity and Activation.
Jumpei Maki, Asami Oshimura, Yudai Shiotani, Maki Yamanaka, Sogen Okuda, Ryo C. Yanagita, Shigeru Kitani, Yasuhiro Igarashi, Yutaka Saito, Yasubumi Sakakibara, Chihiro Tsukano, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2025, 89, 668–679. [DOI: 10.1093/bbb/zbaf008] [Pubmed: 39863420] - Identification of sesquiterpene aldehydes as volatile antifungal compounds in Phaeolepiota aurea culture filtrate.
Kota Seki, Tomoya Tanaka, Emiko Shimoda, Shinji Tanio, Ryo C. Yanagita, Tsugumi Miyazaki, Kento Tokumoto, Toshiaki Tazawa, Kumiko Osaki-Oka, Atsushi Ishihara*.
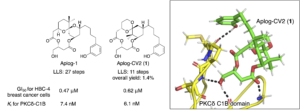
Biosci. Biotechnol. Biochem. 2024, 88, 1395–1402. [DOI: 10.1093/bbb/zbae125] [Pubmed: 39237456] - Effect of phenolic-hydroxy-group incorporation on the biological activity of a simplified aplysiatoxin analog with an (R)-(−)-carvone-based core.
Ryo C. Yanagita*, Yoshiyuki Suzuki, Yasuhiro Kawanami, Yusuke Hanaki, Kazuhiro Irie.
Biosci. Biotechnol. Biochem. 2024, 88, 992–998. [DOI: 10.1093/bbb/zbae091] [Pubmed: 38936828] [Accepted Manuscript ]
]
- A new labdane-type diterpenoid from leaves of Vitex rotundifolia.

Yusuke Hanaki*, Rintaro Abe, Yasunori Sugiyama, Yasumasa Hara, Ryo C. Yanagita.
Results Chem. 2024, 7, 101512. [DOI: 10.1016/j.rechem.2024.101512] - Biological evaluation of a phosphate ester prodrug of 10-methyl-aplog-1, a simplified analogue of aplysiatoxin, as a possible latency-reversing agent for HIV reactivation.
Jumpei Maki, Yusuke Hanaki, Ryo C. Yanagita, Masayuki Kikumori, Anastasiia Kovba, Ayaka Washizaki, Chihiro Tsukano, Hirofumi Akari, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2023, 87, 1453–1461. [DOI: 10.1093/bbb/zbad128] [Pubmed: 37682524] - Cell Morphology-Based Screening Identified Vitetrifolin D from Vitex rotundifolia as an Inhibitor of Phorbol Ester–Induced Downregulation of E-Cadherin in HHUA Endometrial Cells.

Yusuke Hanaki*, Nichika Iwase, Yasunori Sugiyama, Sena Miyoshi, Ryo C. Yanagita.
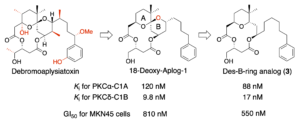
BPB Reports 2023, 6, 103–107. [DOI: 10.1248/bpbreports.6.3_103] - A simplified analog of debromoaplysiatoxin lacking the B-ring of spiroketal moiety retains protein kinase C-binding and antiproliferative activities.
Tomoki Sekido, Kosuke Yamamoto, Ryo C. Yanagita*, Yasuhiro Kawanami, Yusuke Hanaki, Kazuhiro Irie.
Bioorg. Med. Chem. 2022, 73, 116988. [DOI: 10.1016/j.bmc.2022.116988] [Pubmed: 36113282] [Accepted Manuscript ]
]
- 12-O-Tetradecanoylphorbol 13-acetate promotes proliferation and epithelial–mesenchymal transition in HHUA cells cultured on collagen type I gel: A feasible model to find new therapies for endometrial diseases.
Yusuke Hanaki*, Sena Miyoshi, Yasunori Sugiyama, Ryo C. Yanagita, Masashi Sato.
Biosci. Biotechnol. Biochem. 2022, 86, 1417–1422. [DOI: 10.1093/bbb/zbac136] [Pubmed: 35973688] - Synthesis and Characterization of Propeller- and Parallel-Type Full-Length Amyloid β40 Trimer Models.
Ayumi Uchino, Yumi Irie, Chihiro Tsukano, Taiji Kawase, Kenji Hirose, Yusuke Kageyama, Ikuo Tooyama, Ryo C. Yanagita, Kazuhiro Irie*.
ACS Chem. Neurosci. 2022, 13, 2517–2528. [DOI: 10.1021/acschemneuro.2c00363] [Pubmed: 35930616] - Structural basis of the 24B3 antibody against the toxic conformer of amyloid β with a turn at positions 22 and 23.
Yumi Irie, Yuka Matsushima, Akiko Kita, Kunio Miki, Tatsuya Segawa, Masahiro Maeda, Ryo C. Yanagita, Kazuhiro Irie*.
BIochem. Biophys. Res. Commun. 2022, 621, 162–167. [DOI: 10.1016/j.bbrc.2022.07.010] [Pubmed: 35839743] - Design, synthesis, and biological activity of synthetically-accessible analog of aplysiatoxin with (R)-(−)-carvone-based conformation-controlling unit.
Yoshiyuki Suzuki, Keiichi Moritoki, Mizuki Kajiwara, Ryo C. Yanagita*, Yasuhiro Kawanami, Yusuke Hanaki, Kazuhiro Irie.
Biosci. Biotechnol. Biochem. 2022, 86, 1013–1023. [DOI: 10.1093/bbb/zbac084] [Pubmed: 35648459] [Accepted Manuscript ]
]
- AI and computational chemistry-accelerated development of an alotaketal analogue with conventional PKC selectivity.
Jumpei Maki, Asami Oshimura, Chihiro Tsukano, Ryo C. Yanagita, Yutaka Saito, Yasubumi Sakakibara, Kazuhiro Irie*.
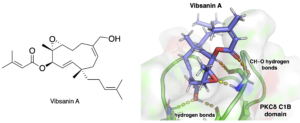
Chem. Commun. 2022, 58, 6693–6696. [DOI: 10.1039/D2CC01759H] [Pubmed: 35608215] - Analysis of binding mode of vibsanin A with protein kinase C C1 domains: An experimental and molecular dynamics simulation study.
Ryo C. Yanagita*, Mao Otani, Satoshi Hatanaka, Hiroto Nishi, Shota Miyake, Yusuke Hanaki, Masashi Sato, Yasuhiro Kawanami, Kazuhiro Irie.
J. Mol. Struct. 2022, 1260, 132866. [DOI: 10.1016/j.molstruc.2022.132866] [Accepted Manuscript ]
]
- Structure optimization of the toxic conformation model of amyloid β42 by intramolecular disulfide bond formation.
Yuka Matsushima, Yumi Irie, Yusuke Kageyama, Jean-Pierre Bellier, Ikuo Tooyama, Takahito Maki, Toshiaki Kume, Ryo C. Yanagita, Kazuhiro Irie*.
ChemBioChem 2022, 23, e202200029. [DOI: 10.1002/cbic.202200029] [Pubmed: 35165998] - Evaluation of the in vitro cytotoxicity of oscillatoxins E and F under nutrient-starvation culture conditions.

Yusuke Hanaki*, Yusuke Araki, Toshio Nishikawa, Ryo C. Yanagita.
Fundam. Toxicol. Sci. 2021, 8, 69–73. [DOI: 10.2131/fts.8.69] - Effects of side chain length of 10-methyl-aplog-1, a simplified analog of debromoaplysiatoxin, on PKC binding, anti-proliferative, and pro-inflammatory activities.
Atsuko Gonda, Koji Takada, Ryo C. Yanagita, Shingo Dan, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2021, 85, 168–180. [DOI: 10.1093/bbb/zbaa024] [Pubmed: 33577665] - Synthesis and biological activities of simplified aplysiatoxin analogs focused on the CH/π interaction.
Takumi Kobayashi, Ryo C. Yanagita, Kazuhiro Irie*.
Bioorg. Med. Chem. Lett. 2021, 30, 127657. [DOI: 10.1016/j.bmcl.2020.127657] [Pubmed: 33130291] - Evaluation of the Anti-Proliferative Activity of Rare Aldohexoses against MOLT-4F and DU-145 Human Cancer Cell Line and Structure-Activity Relationship of D-Idose.

Hironobu Ishiyama, Ryo C. Yanagita*, Kazune Takemoto, Natsumi Kitaguchi, Yuuki Uezato, Yasunori Sugiyama, Masashi Sato, Yasuhiro Kawanami.
J. Appl. Glycosci. 2020, 67, 95–101. [DOI: 10.5458/jag.jag.JAG-2020_0006] [Pubmed: 34354535] - Control of the toxic conformation of amyloid β42 by intramolecular disulfide bond formation.
Yuka Matsushima, Ryo C. Yanagita, Kazuhiro Irie*.
Chem. Commun. 2020, 56, 4118–4121. [DOI: 10.1039/D0CC01053G] [Pubmed: 32163091] - Nematocidal Activity of 6-O-octanoyl- And 6-O-octyl-D-allose Against Larvae of Caenorhabditis elegans.
Hirofumi Sakoguchi, Tomoya Shintani, Hironobu Ishiyama, Ryo C. Yanagita, Yasuhiro Kawanami, Masashi Sato*.
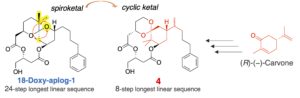
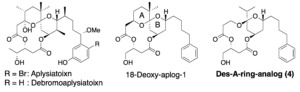
Biosci. Biotechnol. Biochem. 2019, 83 (12), 2194–2197. [DOI: 10.1080/09168451.2019.1648206] [Pubmed: 31357905] - Synthesis, Conformation, and Biological Activities of a Des-A-Ring Analog of 18-Deoxy-aplog-1, a Simplified Analog of Debromoaplysiatoxin.
Yoshiki Ashida, Ryo C. Yanagita*, Yasuhiro Kawanami, Mutsumi Okamura, Shingo Dan, Kazuhiro Irie.
Heterocycles 2019, 99 (2), 942–957. [DOI: 10.3987/COM-18-S(F)60] [CLOCKSS ] [Accepted Manuscript
] [Accepted Manuscript ] [SI
] [SI ]
]
- Synthesis and biochemical characterization of quasi-stable trimer models of full-length amyloid β40 with a toxic conformation.
Yumi Irie, Mizuho Hanaki, Kazuma Murakami, Tsuneo Imamoto, Takumi Furuta, Takeo Kawabata, Taiji Kawase, Kenji Hirose, Yoko Monobe, Ken-ichi Akagi, Ryo C. Yanagita, Kazuhiro Irie*.
Chem. Commun. 2019, 55 (2), 182–185. [DOI: 10.1039/C8CC08618D] [Pubmed: 30519688] - Synthesis and Biological Activities of Acetal Analogs at Position 3 of 10-Methyl-Aplog-1, a Potential Anti-Cancer Lead Derived from Debromoaplysiatoxin.
Koutaro Hayakawa, Yusuke Hanaki, Harukuni Tokuda, Ryo C. Yanagita, Yu Nakagawa, Mutsumi Okamura, Shingo Dan, Kazuhiro Irie*.
Heterocycles 2018, 97 (1), 478–492. [DOI: 10.3987/COM-18-S(T)37] [CLOCKSS ] [Accepted Manuscript
] [Accepted Manuscript ] [SI
] [SI ]
] - Synthesis and inhibitory activity of deoxy-D-allose amide derivative against plant growth.
Md. Tazul Islam Chowdhury, Hikaru Ando, Ryo C. Yanagita, Yasuhiro Kawanami*.
Biosci. Biotechnol. Biochem. 2018, 82 (5), 775–779. [DOI: 10.1080/09168451.2018.1445521] [Pubmed: 29513080] - Loss of the phenolic hydroxyl group and aromaticity from the side chain of anti-proliferative 10-methyl-aplog-1, a simplified analog of aplysiatoxin, enhances its tumor-promoting and proinflammatory activities.

Yusuke Hanaki, Masayuki Kikumori, Harukuni Tokuda, Mutsumi Okamura, Shingo Dan, Naoko Adachi, Naoaki Saito, Ryo C. Yanagita, Kazuhiro Irie*.
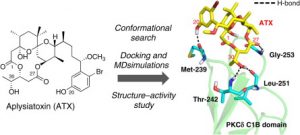
Molecules 2017, 22 (4), E631. [DOI: 10.3390/molecules22040631] [Pubmed: 28406454] - Binding mode prediction of aplysiatoxin, a potent agonist of protein kinase C, through molecular simulation and structure–activity study on simplified analogs of the receptor-recognition domain.
Yoshiki Ashida, Ryo C. Yanagita*, Chise Takahashi, Yasuhiro Kawanami, Kazuhiro Irie.
Bioorg. Med. Chem. 2016, 24 (18), 4218-4227. [DOI: 10.1016/j.bmc.2016.07.011] [Pubmed: 27436807] [Accepted Manuscript ]
]
- Syntheses and biological activities of deoxy-D-allose fatty acid ester analogs.
Md. Tazul Islam Chowdhury, Hikaru Ando, Ryo C. Yanagita, Yasuhiro Kawanami*.
Biosci. Biotechnol. Biochem. 2016, 80 (4), 676-681. [DOI: 10.1080/09168451.2015.1132151] [Pubmed: 26822163] - Structural optimization of 10-methyl-aplog-1, a simplified analog of debromoaplysiatoxin, as an anticancer lead.
Masayuki Kikumori, Ryo C. Yanagita, Harukuni Tokuda, Kiyotake Suenaga, Hiroshi Nagai, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2016, 80 (2) 221-231. [DOI: 10.1080/09168451.2015.1091718] [Pubmed: 26452398] - Synthesis and biological activities of the amide derivative of aplog-1, a simplified analog of aplysiatoxin with anti-proliferative and cytotoxic activities.
Yusuke Hanaki, Ryo C. Yanagita, Takahiro Sugahara, Misako Aida, Harukuni Tokuda, Nobutaka Suzuki, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2015, 79 (6) 888-895. [DOI: 10.1080/09168451.2014.1002452] [Pubmed: 25612633] - Effect of BF3 on the enantioselective reduction of trifluoromethyl ketones using a chiral lactam alcohol with borane.
Yuki Harauchi, Chihiro Takakura, Toshio Furumoto, Ryo C. Yanagita, Yasuhiro Kawanami*.
Tetrahedron: Asymmetry 2015, 26 (7) 333-337. [DOI: 10.1016/j.tetasy.2015.02.011] - Synthesis of 6-O-decanoyl-D-altrose and 6-O-decanoyl-D-gulose and evaluation of their biological activity on plant growth.
Md. Tazul Islam Chowdhury, Madoka Naito, Ryo C. Yanagita, Yaushiro Kawanami*.
Plant Growth Regul. 2015, 75 (3) 707-713. [DOI: 10.1007/s10725-014-9972-2] - Improved and large-scale synthesis of 10-methyl-aplog-1, a potential lead for an anticancer drug.
Masayuki Kikumori, Ryo C. Yanagita, Kazuhiro Irie*.
Tetrahedron 2014, 70 (52) 9776-9782. [DOI: 10.1016/j.tet.2014.11.026] - Anti-proliferative activity of 6-O-acyl-D-allose against the human leukemia MOLT-4F cell line.
Ryo C. Yanagita*, Katsuya Kobashi, Chisa Ogawa, Yoshiki Ashida, Haruka Yamaashi, Yasuhiro Kawanami.
Biosci. Biotechnol. Biochem. 2014, 78 (2) 190-194. [DOI: 10.1080/09168451.2014.882747] [Pubmed: 25036670]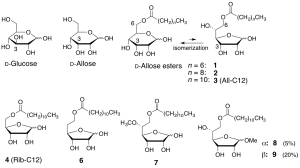
- Effects of two sulfated triterpene saponins echinoside A and holothurin A on the inhibition of dietary fat absorption and obesity reduction.
Yuming Wang, Jiahui Wang, Ryo C. Yanagita, Chunhua Liu, Xiaoqian Hu, Ping Dong, Changhu Xue*, Yong Xue*.
Biosci. Biotechnol. Biochem. 2014, 78 (1) 139-146. [DOI: 10.1080/09168451.2014.877830] [Pubmed: 25036496] - Effects of the methoxy group in the side chain of debromoaplysiatoxin on its tumor-promoting and anti-proliferative activities.
Ryo C. Yanagita, Hiroaki Kamachi, Masayuki Kikumori, Harukuni Tokuda, Nobutaka Suzuki, Kiyotake Suenaga, Hiroshi Nagai, Kazuhiro Irie*.
Bioorg. Med. Chem. Lett. 2013, 23 (15) 4319-4323. [DOI: 10.1016/j.bmcl.2013.05.096] [Pubmed: 23803585]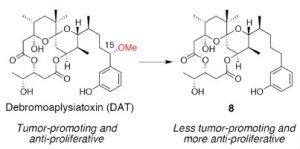
- Structure–activity studies on the side chain of a simplified analog of aplysiatoxin (aplog-1) with anti-proliferative activity.
Hiroaki Kamachi, Keisuke Tanaka, Ryo C. Yanagita, Akira Murakami, Kazuma Murakami, Harukuni Tokuda, Nobutaka Suzuki, Yu Nakagawa, Kazuhiro Irie*.
Bioorg. Med. Chem. 2013, 21 (10) 2695-2702. [DOI: 10.1016/j.bmc.2013.03.013] [Pubmed: 23582444] - Synthesis of antineoplastic analogs of aplysiatoxin with various side chain structures.
Yuki Shu, Ryo C. Yanagita, Harukuni Tokuda, Nobutaka Suzuki, Kazuhiro Irie*.
Heterocycles 2012, 86 (1) 281-303. [DOI: 10.3987/COM-12-S(N)8] [CLOCKSS ] [Accepted Manuscript
] [Accepted Manuscript ]
] - Identification and biolofical activities of bryostatins from Japanese bryozoan.
Sayo Ueno. Ryo C. Yanagita, Kazuma Murakami, Akira Murakami, Harukuni Tokuda, Nobutaka Suzuki, Takeshi Fujiwara, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2012, 76 (5) 1041-1043. [DOI: 10.1271/bbb.120026] [Pubmed: 22738985] - Structure–activity studies on the spiroketal moiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity.
Masayuki Kikumori. Ryo C. Yanagita, Harukuni Tokuda, Nobutaka Suzuki, Hiroshi Nagai, Kiyotake Suenaga, Kazuhiro Irie*.
J. Med. Chem. 2012, 55 (11) 5614-5626. [DOI: 10.1021/jm300566h] [Pubmed: 22625994] - Synthesis and structure–activity studies of simplified analogues of aplysiatoxin with antiproliferative activity like bryostatin-1.
Kazuhiro Irie*, Masayuki Kikumori, Hiroaki Kamachi, Keisuke Tanaka, Akira Murakami, Ryo C. Yanagita, Harukuni Tokuda, Nobutaka Suzuki, Hiroshi Nagai, Kiyotake Suenaga, Yu Nakagawa.
Pure Appl. Chem. 2012, 84 (6) 1341-1351. [DOI: 10.1351/PAC-CON-11-08-22] - Enantioselective reduction of α,β-enones using an oxazaborolidine catalyst generated in situ from a chiral lactam alcohol.
Yasuhiro Kawanami*, Yudai Mikami, Kazuya Kiguchi, Yuki Harauchi, Ryo C. Yanagita.
Tetrahedron: Asymmetry 2011, 22 (20—22), 1891–1894. [DOI: 10.1016/j.tetasy.2011.10.018] - Generation of ‘unnatural natural product’ library and identification of a small molecule inhibitor of XIAP.
Tatsuro Kawamura, Kohei Matsubara, Hitomi Otaka, Etsu Tashiro, Kazutoshi Shindo, Ryo C. Yanagita, Kazuhiro Irie, Masaya Imoto*.
Bioorg. Med. Chem. 2011, 19 (14), 4377–4385. [DOI: 10.1016/j.bmc.2011.05.009] [PubMed: 21696964] - Synthesis and biological evaluation of the 12,12-dimethyl derivative of aplog-1, an anti-proliferative analog of tumor-promoting aplysiatoxin.
Yu Nakagawa, Masayuki Kikumori, Ryo C. Yanagita, Akira Murakami, Harukuni Tokuda, Hiroshi Nagai, Kazuhiro Irie*.
Biosci. Biotechnol. Biochem. 2011, 75 (6), 1167–1173. [DOI: 10.1271/bbb.110130] [PubMed: 21670518] - Role of the phenolic hydroxyl Group in the biological activities of simplified analogue of aplysiatoxin with antiproliferative activity.
Ryo C. Yanagita, Hiroaki Kamachi, Keisuke Tanaka, Akira Murakami, Yu Nakagawa, Harukuni Tokuda, Hiroshi Nagai, Kazuhiro Irie*.
Bioorg. Med. Chem. Lett. 2010, 20 (20), 6064–6066. [DOI: 10.1016/j.bmcl.2010.08.051] [PubMed: 20817520]
Non-Peer-Reviewed Papers
- Inhibitory activity of 6-O-decyl-D-allose and 6-(decanoylamino)-6-deoxy-D-allose against plant growth.
Haruka Yamaashi, Md. Tazul Islam Chowdhury, Ryo C. Yanagita, Yasuhiro Kawanami.
Tech. Bull. Fac. Agr. Kagawa Univ. 2017, 69 (122), 17–22.
Book Chapters and Reviews
- Practical Enantioselective Reduction of Ketones Using Oxazaborolidine Catalysts Generated In Situ from Chiral Lactam Alcohols.

Yasuhiro Kawanami*, Ryo C. Yanagita.
Molecules 2018, 23 (10), E2408. [DOI: 10.3390/molecules23102408] [Pubmed: 30241305] - Synthesis and biological activities of simplified analogs of the natural PKC ligands, bryostatin-1 and aplysiatoxin.
Kazuhiro Irie*, Ryo C. Yanagita.
Chem. Rec. 2014, 14 (2), 251–267. [DOI: 10.1002/tcr.201300036] [PubMed: 24677503] - Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism for protein kinase Cδ.
Kazuhiro Irie*, Ryo C. Yanagita, Yu Nakagawa.
Med. Res. Rev. 2012, 32 (3), 518–535. [DOI: 10.1002/med.20220] [PubMed: 21064191]
Archives for pre-2009 papers
Peer-Reviewed Papers
*corresponding author
- Binding selectivity of 1- or 12-substituted indolactam derivatives for protein kinase C isozymes.
Ryo C. Yanagita, Keiji Torii, Yu Nakagawa, Kazuhiro Irie*.
Heterocycles 2007, 73 (1), 289–302. [DOI: 10.3987/COM-07-S(U)3] [CLOCKSS ] [Accepted Manuscript
] [Accepted Manuscript ]
]